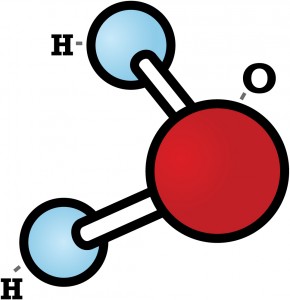
Did you know that while no two snowflakes are exactly the same, they are all six-sided? Snowflakes’ hexagonal shapes are due to the molecular structure of ice. As you might know, each water molecule is made of two hydrogen atoms bonded to one oxygen atom and looks something like this:
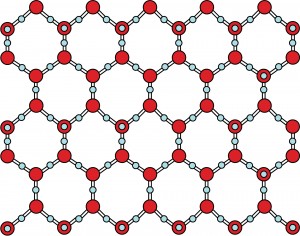
As water freezes, the molecules are forced to align themselves into a very particular structure – a hexagonal lattice that is the basis for the six-sided snowflakes.
This shape also helps explain why water expands as it freezes, while all other solids contract. Ice takes up more space as a solid and is therefore less dense, meaning that ice floats in liquid water. No other compound behaves this way!
I have found a contemporary snowflake Bentley in Kenneth G. Libbrecht of Caltech and his webpage SnowCrystals.com
is full of excellent resources. Here are some links to his site:
Both .gifs above are courtesy of Mr. Kenneth G. Libbrecht